Allergy immunotherapy patients in real clinical practice: The REACT cohort
The REACT (REAl world effeCtiveness of allergy immunoTherapy) study included a total of 92,048 patients with allergic rhinitis (AR) with or without asthma. Half of the cohort was treated with allergy immunotherapy (AIT). Read more to learn about the characteristics of these real-life patients.
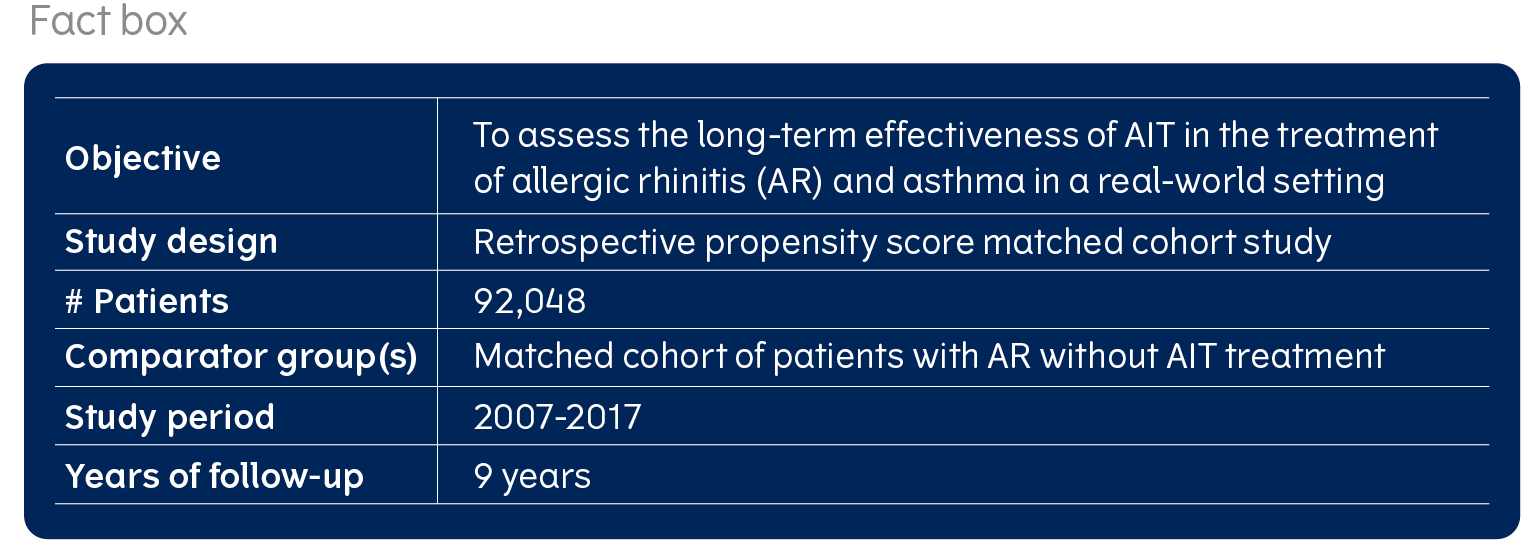
The REACT study used insurance claims data from a large, representative, German health insurance database. The database gave access to up to 5.9 million anonymised individuals per year. During the observation period of 2007-2017, more than 1 million individuals with a diagnosis of AR were identified as eligible for the study, of which, 47,440 were enrolled in the study based on receiving treatment with AIT.
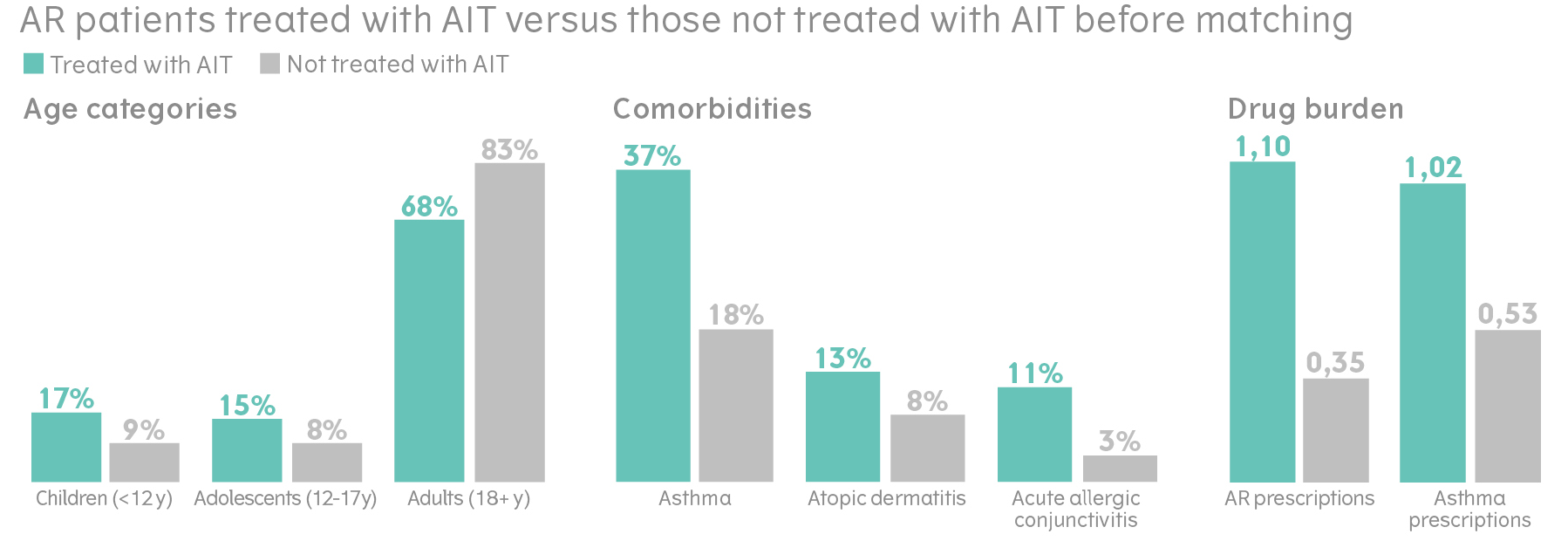
When comparing the group of patients with AR who were treated with AIT, with those who had not, the groups differ in many key patient characteristics. In the group treated with AIT, a larger proportion of patients were children and adolescents. The AIT group also had more allergic comorbidities.
To ensure a good basis for comparison, AIT subjects were matched with AR subjects not treated with AIT using a method called propensity score matching (PSM) (read more here). The AIT patients were matched 1:1 with those having an AR diagnosis and not receiving AIT treatment on a wide range of variables, such as age, comorbidities, AR and asthma prescriptions and asthma treatment steps. This resulted in a cohort of 46,025 AIT patients and an equal number of controls.
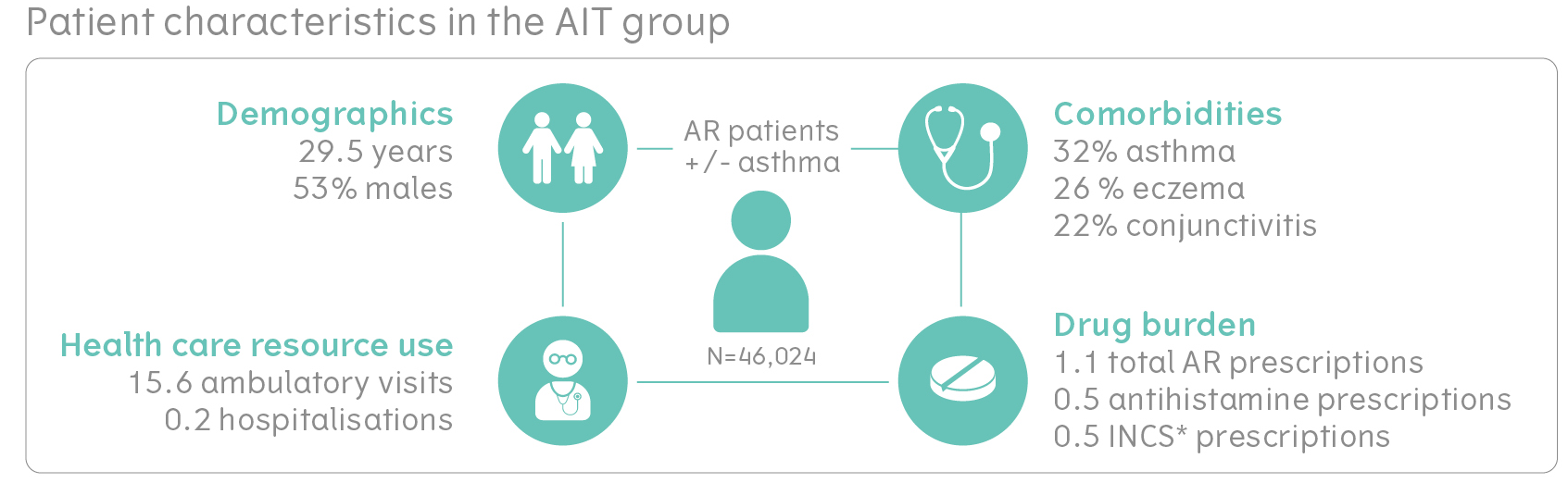
On average AR+/- asthma patients in the study were 29.5 years old and had a high prevalence of comorbidities
The AIT group had a mean age of 29.5 years, 53% were males, and had a high prevalence of comorbidities in the year before starting AIT. They had 1.1 AR prescriptions in the year prior to AIT initiation, of which, the majority were for antihistamines. On average, patients had 15.6 ambulatory visits and 0.2 hospitalisations in the year prior to starting AIT. 32% of them had asthma, 26% had eczema and 22% had conjunctivitis as comorbidities. After successful PSM, groups had similar patient characteristics in the year prior to AIT initiation/ study start. From the index date onwards, AIT patients were exposed to AIT treatment for 549 days, on average.
The study included a range of pre-specified subgroups: Out of the 92,050 patients in the main cohort, 29,228 subjects were included in a pre-existing asthma cohort and 54,274 in a non-asthma cohort. Subgroups by route of administration for AIT comprised N= 36,927 for subcutaneous AIT (SCIT), N=4,816 for sublingual AIT (SLIT)-drops and N=3,754 receiving SLIT-tablets. Subgroups by allergen comprised N=11,713 for grass, N=7,774 for house dust mites (HDM), N=11,897 for tree, and N=9,726 for other allergens.
* Intra-nasal corticosteroid
More on patients included in RCTs for AIT
Click here

Fritzsching B, et al.:” Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: results from the REACT study, a retrospective cohort study” Lancet Regional Health – Europe. 2021; https://doi.org/ 10.1016/j.lanepe.2021.100275



